一种新型通用不对称催化手性双磷配体
时间: 2021-06-10
作者: 百灵威
分享:
迄今为止,科学家已开发出数千种手性双磷配体,其中WingPhos结构独特,在不对称催化中有良好应用前景。WingPhos核心上连接两个蒽基基团,从而构建较深的手性口袋,类似于围绕金属中心的两个翅膀——这正是配体名称的由来。金属中心位于袋的深处,因此可预期底物与蒽基之间的良好相互作用。
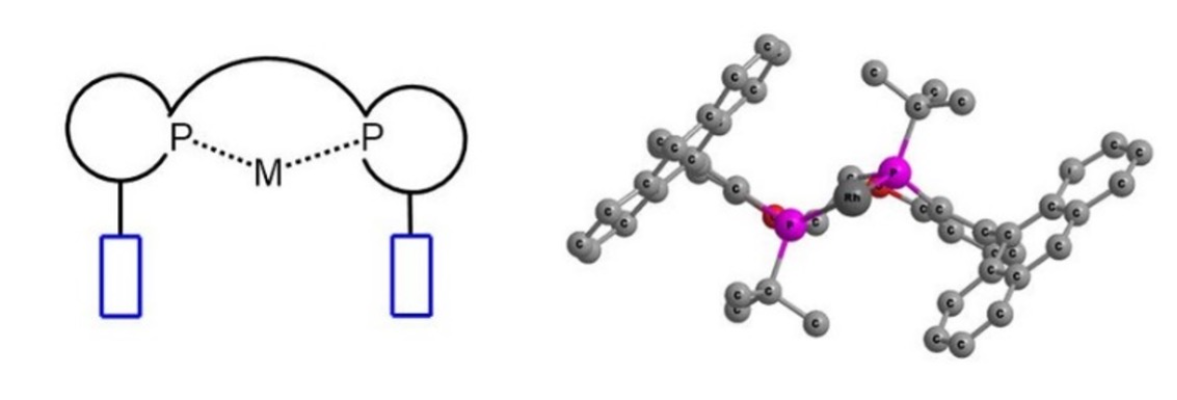
M(Rh)-WingPhos结构
以下是WingPhos可实现的转换示例:
1. 不对称氢化[1]
WingPhos has shown high efficiency in rhodium-catalyzed asymmetric hydrogenation of (E)-β-aryl-N-acetyl enamides, cyclic β-arylenamides, and heterocyclic β-arylenamides. A series of chiral β-arylisopropylamines, 2-aminotetralines, and 3-aminochromans are synthesized in excellent ee's at high substrate to catalyst ratios (showing up to 10,000 TON).
WingPhos在(E)-β-芳基-N-乙酰氨基酰胺、环状β-芳基酰胺和杂环β-芳基酰胺的铑催化不对称氢化反应中显示出很高的效率,可在高底物与催化剂比(高达10,000 TON)时,合成一系列具优异ee值的手性β-芳基异丙基胺、2-氨基四氢呋喃和3-氨基苯并吡喃。
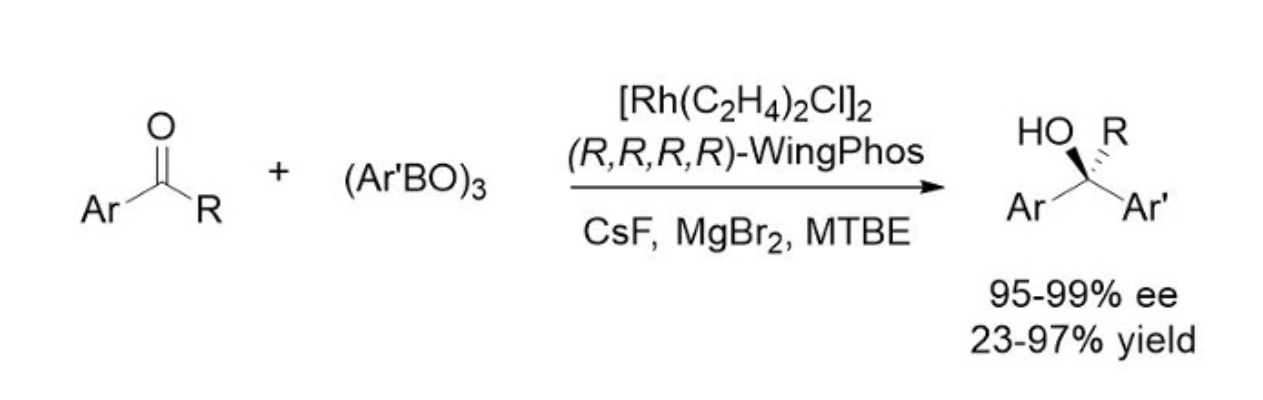
Highly enantioselective additions of arylboroxines to simple aryl ketones have been achieved for the first time with a Rh-WingPhos catalyst, providing a range of chiral diaryl alkyl carbinols in excellent ee's and yields.
Rh-WingPhos催化剂实现了简单芳基酮与芳基硼氧烷的高对映体选择性加成,可生成一系列具有优异ee和收率的手性二芳基烷基甲醇。
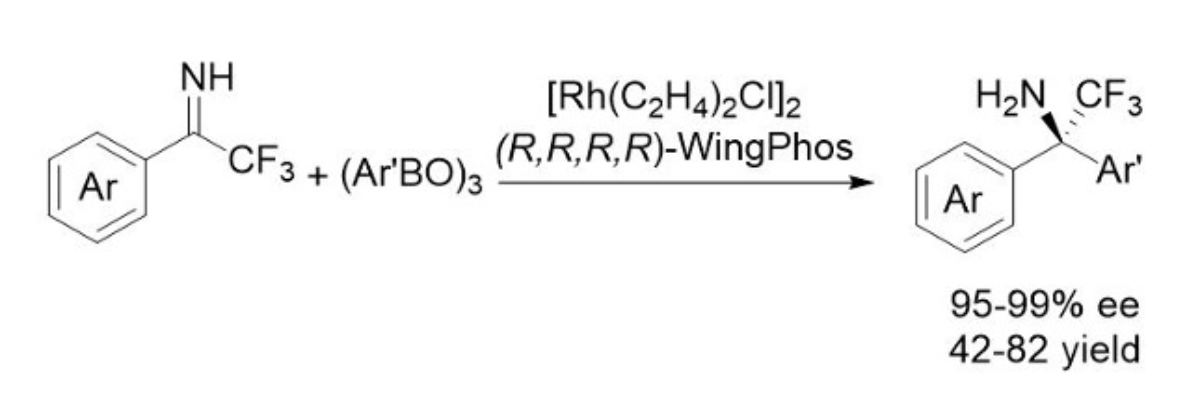
Highly enantioselective rhodium-catalyzed addition of arylboroxines to N-unprotected ketimines are realized by employing WingPhos as the chiral ligand, providing facile and practical access to a range of chiral α-trifluoromethyl-α,α-diaryl amines in excellent ee's and yields with the Rh loading as low as 1 mol %.
通过使用WingPhos手性配体,可以实现芳基硼氧烷向N-保护酮亚胺的高对映选择性铑催化加成反应,从而轻松获得一系列具优异ee和收率的手性α-三氟甲基-α、α-二芳基胺,Rh含量低至1 mol%。
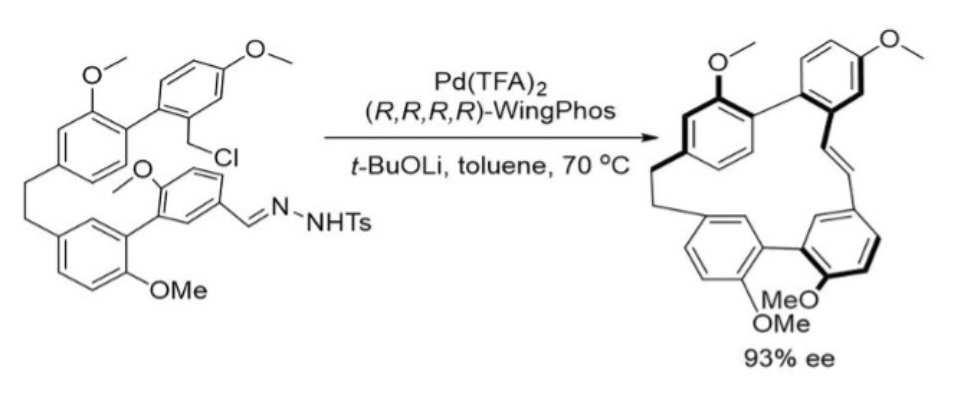
A highly enantioselective macrocyclization between benzyl chloride and a carbene has been realized only with a Pd-WingPhos catalyst by Professor Zhenhua Gu at University of Science and Technology of China, leading to the cyclization product in 93% ee.
中科大的顾振华教授仅使用Pd-WingPhos催化剂,即实现了苄基氯和卡宾之间的高度对映选择性大环化反应,且环化产物的ee高达93%。
![A general and enantioselective palladium-catalyzed tandem allylic substitution of butenylene dicarbonate powered by Wingphos has been developed, forming a series of chiral substituted heterocycles including tetrahydroquinoxalines, piperazines, dihydro-2H-benzo[b][1,4]-oxazine, and morpholines in excellent ee’s and yields.<br><br>](https://shop.jkchemical.com/res/0-6165.jpg)
A general and enantioselective palladium-catalyzed tandem allylic substitution of butenylene dicarbonate powered by Wingphos has been developed, forming a series of chiral substituted heterocycles including tetrahydroquinoxalines, piperazines, dihydro-2H-benzo[b][1,4]-oxazine, and morpholines in excellent ee’s and yields.
Wingphos还可实现对苯二甲酸二丁烯酯的通用和对映选择性钯催化串联烯丙基取代反应,形成一系列手性取代杂环,包括四氢喹喔啉、哌嗪、二氢-2H-苯并[b][1,4]-恶嗪和吗啉,并具有出色的ee和产量。
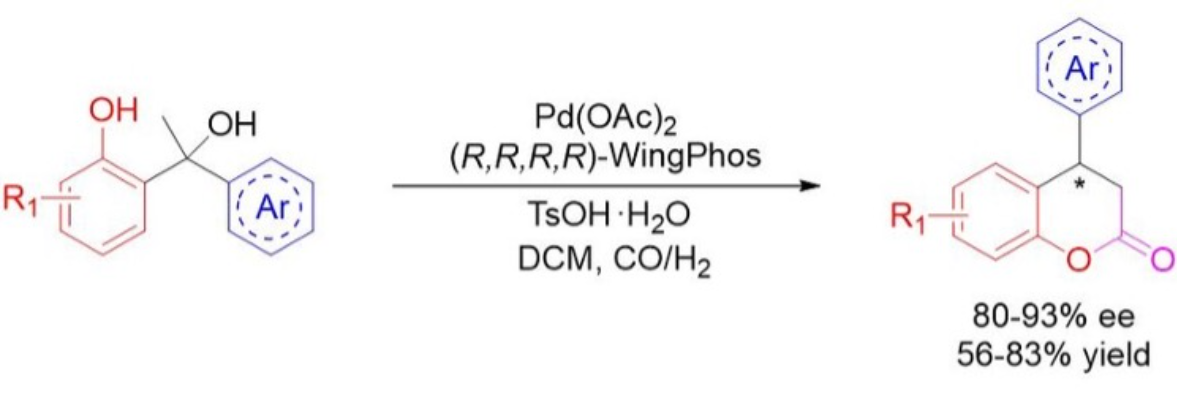
An efficient asymmetric hydroesterfication of diarylmethyl carbinols is developed for the first time with a Pd-WingPhos catalyst, resulting in a series of chiral 4-aryl-3,4-dihydrocoumarins in excellent enantioselectivities and good yields.
二芳基甲基甲醇的有效不对称加氢酯化反应通过Pd-WingPhos催化剂开发成功,获得一系列具有优异对映选择性和良好收率的手性4-芳基-3,4-二氢香豆素。
本文根据中科院上海有机所汤文军教授文章编译。
汤文军教授是具有2,3-二氢苯并[d][1,3]草酰氧磷基团膦配体的发明者,WingPhos属于此类配体,近年来已广泛用于各种不对称转化。更多信息,请阅读The Strem Chemiker Vol. XXXI No. 1
参考文献
- Angew. Chem. Int. Ed. 2013, 52, 4235-4238.
- Angew. Chem. Int. Ed. 2016, 55, 4527-4531.
- Angew. Chem. Int. Ed. 2019, 58, 16119-16123.
- Chin. J. Chem. 2020, 38, 1081-1085.
- Org. Lett. 2020, 22, 4483-4488.
- Angew. Chem. Int. Ed. doi: 10.1002/anie.202015450.
相关产品
品名:
(2R,2'R,3R,3'R)-4,4'-Di(anthracen-9-yl)-3,3'-di-t-butyl-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole, min 98% (>90% ee), [(2R,2'R,3R,3'R)-WingPhos]
(2R,2'R,3R,3'R)-4,4’-二(蒽-9-基)-3,3’-二叔丁基-2,2’,3,3’-四氢-2,2’-二苯并[d][1,3]氧磷酰
CAS:1884680-45-8
货号:15-1970
(2R,2'R,3R,3'R)-4,4'-Di(anthracen-9-yl)-3,3'-di-t-butyl-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole, min 98% (>90% ee), [(2R,2'R,3R,3'R)-WingPhos]
(2R,2'R,3R,3'R)-4,4’-二(蒽-9-基)-3,3’-二叔丁基-2,2’,3,3’-四氢-2,2’-二苯并[d][1,3]氧磷酰
CAS:1884680-45-8
货号:15-1970
品名:
(2S,2'S,3S,3'S)-4,4'-Di(anthracen-9-yl)-3,3'-di-t-butyl-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole, min 98%, (>99% ee), [(2S,2'S,3S,3'S)-WingPhos]
(2S,2'S,3S,3'S)-4,4'-二(蒽-9-基)-3,3'-二叔丁基 - 2,2',3,3'-四氢-2,2-二苯并[d][1,3]氧磷杂环戊烯
CAS:1435940-19-4
货号:15-1975
(2S,2'S,3S,3'S)-4,4'-Di(anthracen-9-yl)-3,3'-di-t-butyl-2,2',3,3'-tetrahydro-2,2'-bibenzo[d][1,3]oxaphosphole, min 98%, (>99% ee), [(2S,2'S,3S,3'S)-WingPhos]
(2S,2'S,3S,3'S)-4,4'-二(蒽-9-基)-3,3'-二叔丁基 - 2,2',3,3'-四氢-2,2-二苯并[d][1,3]氧磷杂环戊烯
CAS:1435940-19-4
货号:15-1975