百灵威Frontier品牌|血红素的生物合成与降解相关试剂
时间: 2023-03-07
作者: 百灵威
分享:
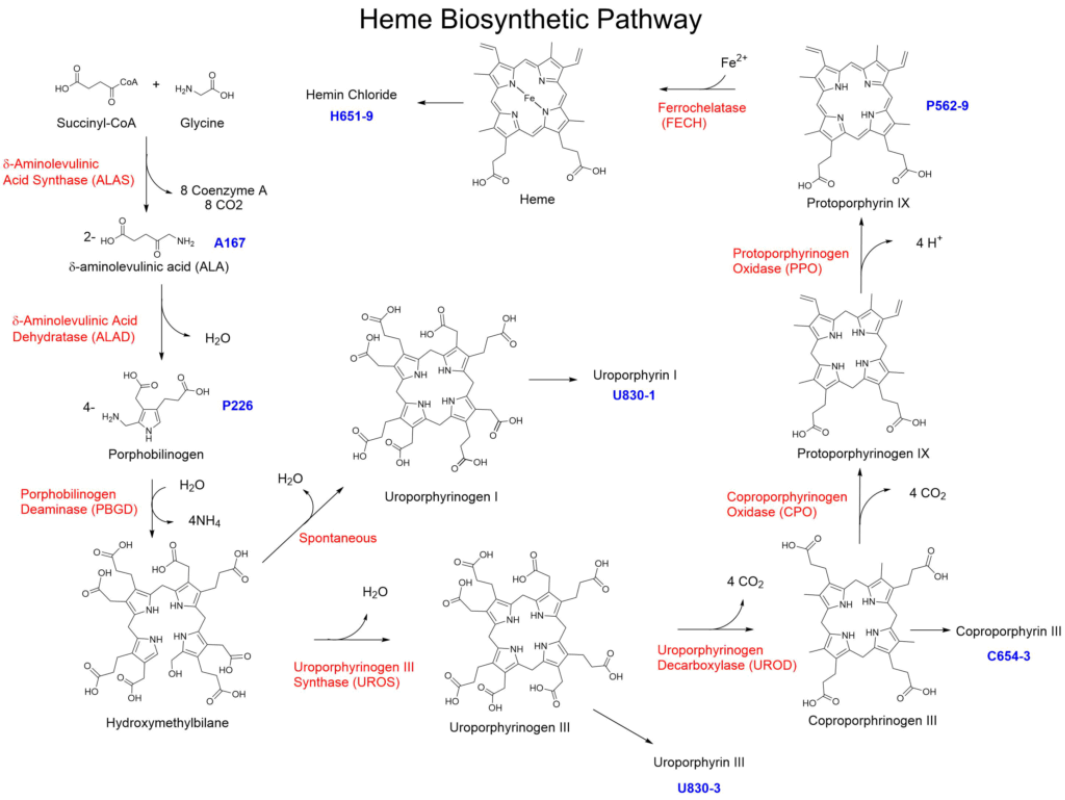
血红素是血红蛋白必不可少的组成部分,其生物合成与降解是生物体中最重要的代谢途径之一。血红素的生物合成过程需要经历八个酶促反应(图1),主要分为以下四个阶段:
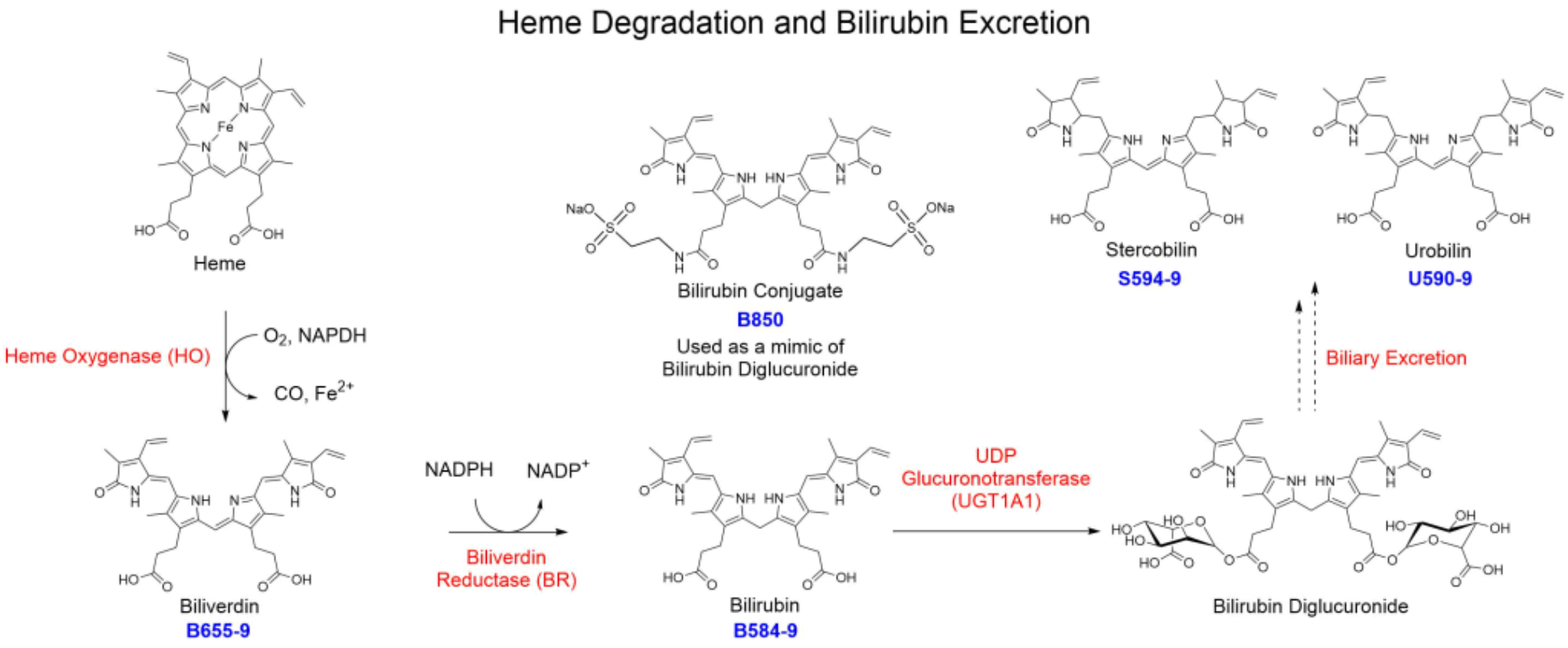
 血红素加氧酶(HO)是血红素降解代谢过程中的限速酶[12]。这种酶能将血红素降解为一氧化碳(CO)、游离铁和胆绿素,其中胆绿素通过胆绿素还原酶(BR)进一步转化为胆红素[13]。胆红素是胆汁中的主要色素,当胆红素进入肝脏后,经UDP葡糖醛酸转移酶(UGT1A1)催化,生成葡萄糖醛酸胆红素[14],最终在肠道微生物作用下,转化成胆素原、粪胆素原和尿胆素原(图2),随粪便排出体外。
血红素加氧酶(HO)是血红素降解代谢过程中的限速酶[12]。这种酶能将血红素降解为一氧化碳(CO)、游离铁和胆绿素,其中胆绿素通过胆绿素还原酶(BR)进一步转化为胆红素[13]。胆红素是胆汁中的主要色素,当胆红素进入肝脏后,经UDP葡糖醛酸转移酶(UGT1A1)催化,生成葡萄糖醛酸胆红素[14],最终在肠道微生物作用下,转化成胆素原、粪胆素原和尿胆素原(图2),随粪便排出体外。
 百灵威可提供10种Frontier品牌血红素的生物合成与降解所需产品,助力该领域科学研究。
百灵威可提供10种Frontier品牌血红素的生物合成与降解所需产品,助力该领域科学研究。
1.δ-氨基乙酰戊酸(ALA)的生成
δ-氨基乙酰丙酸合成酶(ALAS)催化甘氨酸和琥珀酰辅酶A缩合,生成δ-氨基乙酰戊酸(ALA)[1]。2.胆色素原(PBG)的生成
在ALA脱水酶(ALAD)作用下,两分子ALA脱水缩合生成胆色素原(PBG)[2]。3.尿卟啉原Ⅲ(Uro III)和粪卟啉原Ⅲ(Copro III)的生成
四分子PBG经卟啉原脱氨酶(PBGD)催化生成不稳定的羟甲基胆素(HMB)[3],HMB在尿卟啉原III合成酶(UROS)作用下,进一步环化为尿卟啉原Ⅲ(Uro III)[4],当无UROS存在时,HMB会环化为尿卟啉原I[5]。Uro III再经尿卟啉原III脱羧酶(UROD)催化,四个乙酸侧链脱羧变成甲基,生成粪卟啉原III(Copro III)[6]。4.血红素的生成
在粪卟啉原III氧化酶(CPO)作用下,Copro III转化成原卟啉原IX[7,8],再经原卟啉原氧化酶(PPO)催化脱氢生成原卟啉IX(PPIX)[9,10],最后在亚铁螯合酶(FC)催化下和亚铁离子结合,生成血红素[11]。
图1 血红素的生物合成过程

图2 血红素的降解过程

血红素的生物合成相关化合物
由于卟啉原中间体不稳定,在空气中易氧化为相应的卟啉类似物,所以Frontier提供的是与卟啉原相对应的卟啉化合物。| 品名 | CAS | 货号 |
|---|---|---|
| δ-Aminolevulinic acid hydrochloride, >98%
5-氨基乙酰丙酸盐酸盐 | 5451-09-2 | A167 |
| Coproporphyrin III dihydrochloride, >97%
粪卟啉二盐酸盐 | 14643-66-4 | C654-3 |
| Hemin
血红素/卟啉铁 | 16009-13-5 | H651-9 |
| Porphobilinogen, >97%
5-(氨基甲基)-4-(羧基甲基)吡咯-3-丙酸 | 487-90-1 | P226 |
| Protoporphyrin IX, 97%
原卟啉 IX | 553-12-8 | P562-9 |
血红素的降解相关化合物
| 品名 | CAS | 货号 |
|---|---|---|
| Biliverdin hydrochloride
胆绿素盐酸盐 | 856699-18-8 | B655-9 |
| Bilirubin
胆红素 | 635-65-4 | B584-9 |
| Bilirubin conjugate
二牛磺酸胆红素钠盐/缀合胆红素 | 68683-34-1 | B850 |
| Stercobilin hydrochloride (mixture of isomers)
尿胆素盐酸盐 | 34217-90-8 | S594-9 |
| Urobilin hydrochloride (mixture of isomers)
尿胆素Ⅸ盐酸盐 | 28925-89-5 | U590-9 |
参考文献
- Ferreira, G.C., Gong, J. 5-Aminolevulinate synthase and the first step of heme biosynthesis. J Bioenerg Biomembr 27, 151–159 (1995).
- Jaffe, E.K. Porphobilinogen synthase, the first source of Heme’s asymmetry. J Bioenerg Biomembr 27, 169–179 (1995).
- Grandchamp, B., De Verneuil, H., Beaumont, C., Chretien, S., Walter, O. and Nordmann, Y. (1987), Tissue-specific expression of porphobilinogen deaminase. European Journal of Biochemistry, 162: 105-110.
- Battersby, A., Fookes, C., Matcham, G. et al. Biosynthesis of the pigments of life: formation of the macrocycle. Nature 285, 17–21 (1980).
- Paul R. Ortiz de Montellano (2008). “Hemes in Biology”. Wiley Encyclopedia of Chemical Biology. John Wiley & Sons.
- Straka, J.G., Kushner, J.P Purification and characterization of bovine hepatic uroporphyrinogen decarboxylase. Biochemistry 1983, 22, 20, 4664-4672.
- T. Yoshinaga, S. Sano Coproporphyrinogen oxidase: I. Purification, properties, and activation by phospholipids. J. Biol. Chem., 255 (10) (1980), pp. 4722-4726.
- T. Yoshinaga, S. Sano Coproporphyrinogen oxidase: II. Reaction mechanism and role of tyrosine residues on the activity. J. Biol. Chem., 255 (10) (1980), pp. 4727-4731.
- Dailey HA. 1990. Conversion of coproporphyrinogen to protoheme in higher eukaryotes and bacteria: Terminal three enzymes. In Biosynthesis of heme and chlorophylls (ed. Dailey HA), pp. 123–161. McGraw-Hill, New York.
- Dailey, T. A. & Dailey, H. A. Human protoporphyrinogen oxidase: Expression, purification, and characterization of the cloned enzyme. Protein Sci. 5, 98–105 (1996).
- Lecerof, D.; Fodje, M.; Hansson, A.; Hansson, M.; Al-Karadaghi, S. (March 2000). “Structural and mechanistic basis of porphyrin metallation by ferrochelatase”. Journal of Molecular Biology. 297 (1): 221–232.
- Kikuchi G, Yoshida T, Noguchi M (2005). “Heme oxygenase and heme degradation”. Biochem. Biophys. Res. Commun. 338 (1): 558–567.
- Ahmad Z, Salim M, Maines MD (Mar 2002). “Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress”. The Journal of Biological Chemistry. 277 (11): 9226–32..
- Jansen, P.L.M., Mulder, G.J., Burchell, B. and Bock, K.W. (1992), New developments in glucuronidation research: Report of a workshop on “Glucuronidation, its role in health and disease”. Hepatology, 15: 532-544.